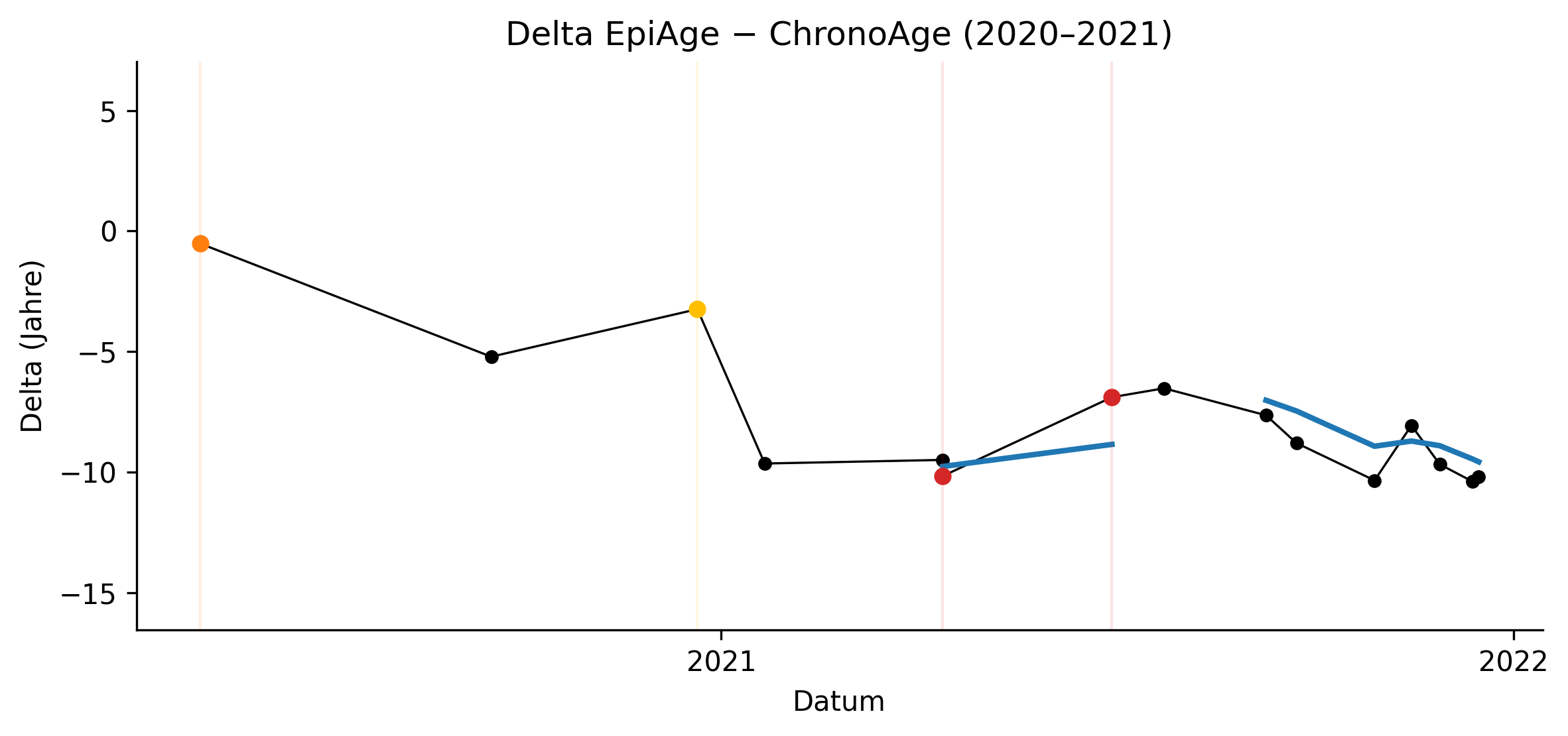
Epigenetic clocks are increasingly applied as biomarkers of biological aging, yet their interpretation is complex in the context of inflammation, lifestyle transitions, and therapeutic interventions. In this six-year longitudinal N-of-1 dataset (n=225 measurements), epigenetic age acceleration (EAA) is best interpreted as a marker of systemic stability rather than an immediate readout of isolated events.
Throughout the entire observation period, a structured rejuvenation protocol was continuously implemented in varying compositions. This multi-component framework included repeated senolytic cycles, NRF2 pathway activation strategies, smoking cessation (June 2022), alcohol cessation (January 2023), and additional undisclosed systemic interventions. The protocol was dynamically adjusted but uninterrupted, indicating that the observed trajectory reflects cumulative systems modulation rather than discrete therapeutic effects.
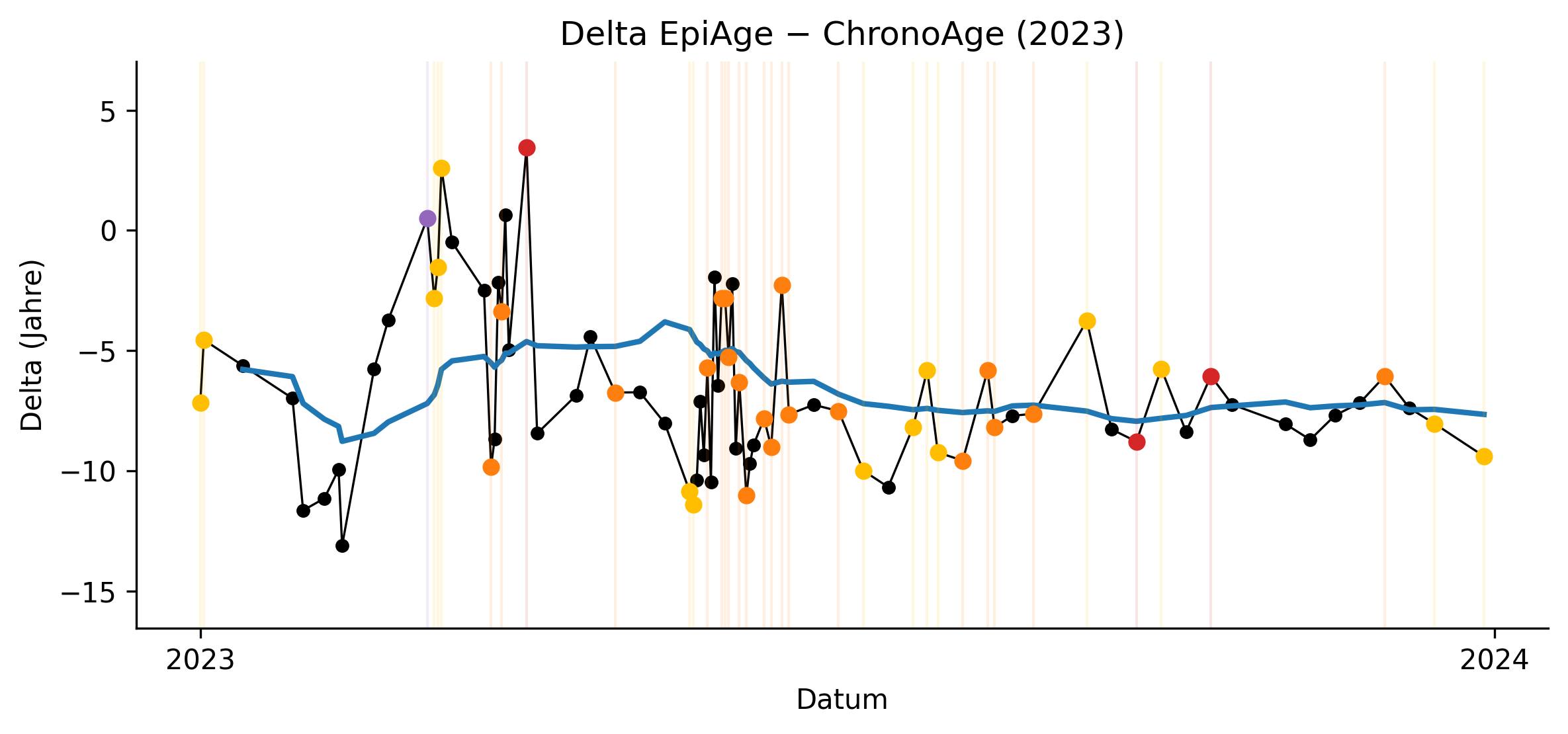
SARS-CoV-2 infections and COVID-19 vaccinations were consistently associated with transient increases in EAA, supporting their interpretation as acute epigenetic stressors. These perturbations were temporally aligned with inflammatory episodes and reproducible in direction. Importantly, after 2023, such events no longer induced sustained upward shifts in the 12-month trajectory, suggesting preserved regenerative capacity rather than cumulative epigenetic damage.
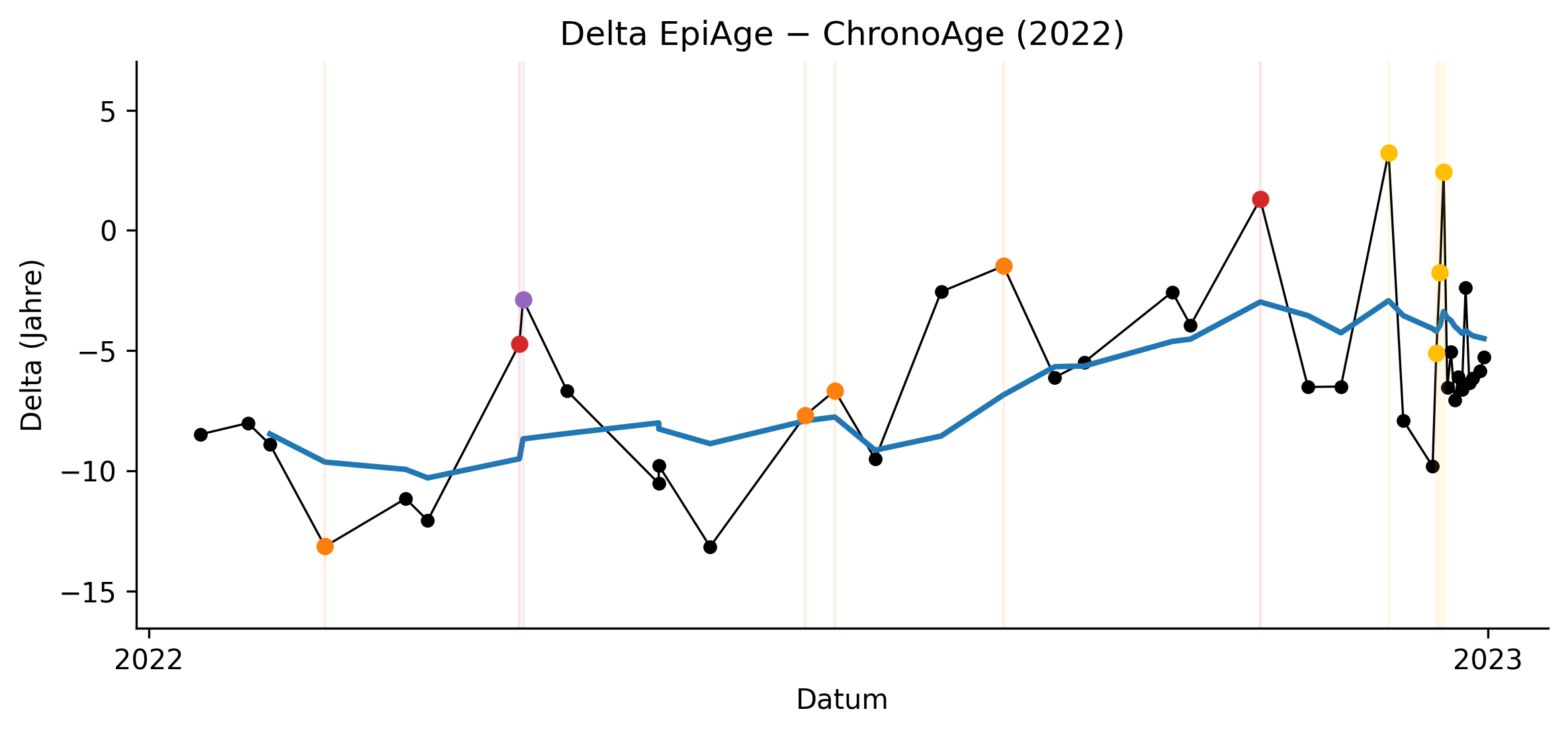
The period from 2022 to early 2023 exhibited maximal EAA variance, coinciding with smoking cessation, subsequent weight gain, alcohol cessation, recurrent infections, dental inflammation, discontinuation of urate-lowering therapy, and repeated senolytic cycles. This systemic reorganization phase was marked by pronounced epigenetic volatility, indicating that clocks are highly sensitive to transitional physiological states but do not reliably reflect directional aging during active restructuring.
Beginning in 2024, a qualitative transition emerged: mean EAA became persistently more negative, variance declined, and values converged toward a stable plateau approximately one decade below chronological age. The most recent measurement represents the most negative EAA value observed in the dataset. This stabilization coincided with independent clinical indicators of reduced inflammatory burden and systemic recovery, including visible improvement of skin quality, emergence of new frontal hair growth, stabilization of benign prostatic enlargement, and sustained metabolic normalization. The concordance between molecular dynamics and phenotypic changes supports the interpretation of a systems-level consolidation rather than transient fluctuation.
Senolytic clusters displayed a biphasic pattern, with short-term EAA elevation followed by delayed reductions below baseline. Repeated cycles did not increase long-term instability; instead, dispersion narrowed over time, consistent with enhanced regulatory resilience.
Measurement robustness strengthens these conclusions. Closely spaced repeat assessments during stable phases showed tight clustering, whereas variability increased in a temporally structured manner during defined stress events. The progressive variance reduction after 2024 argues against stochastic assay noise and supports biological relevance.
Further analyses are underway, including integration with genomic risk profiling, chromatin-state measurements, circulating glycan age testing, and environmental burden assessments (including microplastic exposure). In addition, a second longitudinal dataset from a female participant following a comparable intervention framework is being collected for cross-individual validation. These extensions will allow assessment of reproducibility, mechanistic coherence, and sex-specific dynamics.
Collectively, these findings support a threshold-based systems model in which epigenetic age reflects the emergent state of a biological network once cumulative inflammatory and senescent burdens fall below a critical level. Durable rejuvenation manifests as reduced variance and stabilization around a lower epigenetic set-point rather than linear age reversal.

 Back to all posts
Back to all posts